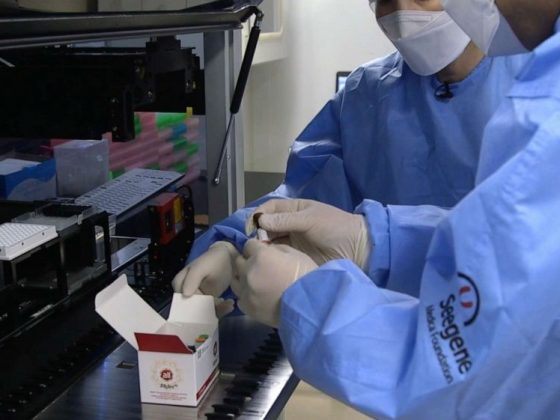
Five Korean companies have manufactured testing kits from a World Health Organization formulation, and as a result, the country quickly developed a system that could assess about 430,000 people a week. South Korea took a risk, releasing briskly vetted tests, then circling back later to spot check their effectiveness. As of now, South Korea has received requests from 121 countries to export or donate coronavirus testing supplies. The following are the companies that produce testing kits (Company Name-Testing Kit Name): Seegene-Allplex, Kogene Biotech-Power Check, Solgent-DiaPlex Q, SD Biosensor-Standard M, and BioSewoom-Real-Q.
FEATURES
- The testing kits are based on the real-time polymerase chain reaction (RT-PCR) testing method, in which results can come out in six hours.
- The companies now produce enough diagnostic reagents to test 135,000 people a day.
- Seegene’s kit can perform 1,000 tests simultaneously and deliver the diagnosis in less than four hours.
- Solgent’s kit can come up with a diagnosis in under two hours.
ONGOING RESULTS
- The urgent use approval system made possible for the companies to get approval in just one week and supply the testing kits.
- Extensive testing is said to have controlled the rise of COVID-19 patients in South Korea.
- While the kits are already being exported to eight countries including UAE, Germany, India, and Italy, South Korea has received requests from 121 countries to export or donate coronavirus testing supplies.
- Due to high demand, Seoul has set up a task force with online updates and contact information of 29 Korean manufacturers and exporters specializing in COVID-19 diagnostic devices.
- 3 Korean diagnostic kits have received FDA approval, and other test kits are expected to receive emergency use approval from the FDA.
- Low fatality rate: about 0.9%
Sources: ABC News, Forbes, NY Times, Reuters